INTRODUCTION
Outcomes of acute Myeloid Leukemia (AML) progressed from JAK2-mutant ( JAK2-mut) myeloproliferative neoplasms (MPNs) (post-MPN AML) are dismal, with limited responsiveness to cytotoxic therapies, hypomethylating agents, BCL-2 inhibitor or JAK2 inhibitor, with a median overall survival of less than 1 year ( Dunbar, et al. Blood, 2020).
BCL-xL has been found to be overexpressed in cells from JAK2-mut MPN patients ( Petiti et al. J Cell Mol Med, 2020), thus making it a potential therapeutic target. However, the clinical utility of navitoclax, a BCL-xL and BCL-2 dual inhibitor, as demonstrated in combination with JAK2 inhibitor ruxolitinib in patients with myelofibrosis ( Harrison et al. J Clin Oncol, 2022), has been notable for an on-target thrombocytopenia due to the essential role of BCL-xL in platelet survival. In contrast, DT2216, a VHL-recruiting BCL-xL PROTAC, exhibits potent degradation of BCL-xL in leukemia cells while sparing platelets due to minimal expression of VHL in platelets ( Khan et.al Nat. Med, 2019). Consequently, DT2216 may represent an attractive strategy to target BCL-xL, reducing the apoptotic threshold and increasing responsiveness to chemotherapy selectively in tumor cells. In this study, we evaluated the pre-clinical efficacy of DT2216 in combination with ruxolitinib, 5-azacytidine (AZA), or MCL-1 inhibitor S63845 in JAK2-mut AML models.
METHODS
To validate the dependency of BCL2L1 (gene encoding BCL-xL) on JAK2-mut AML, we analyzed genome-wide CRISPR screen data, RNA-seq data from the DepMap portal and Beat AML 1.0 cohort. To assess the efficacy of DT2216-based treatments, including DT2216 alone, DT2216/AZA combination, DT2216/ruxolitinib combination, and DT2216/S63845 combination, we conducted the Cell Titer Glo (CTG) assay using JAK2-mut AML cell lines and primary leukemia samples. Since most JAK2-mut AML patients are resistant to ruxolitinib, we included ruxolitinib-resistant (ruxo-re) cell lines. The combination index (CI) values obtained at the ED 50, ED 75, and ED 90 were used to determine synergism (CI <1) between the agents, with lower values indicating stronger synergism. Clonogenic assays with three distinct JAK2-mut AML patient samples were performed using DT2216 and/or AZA to assess the anti-leukemia effects on hematopoietic stem and progenitor cells (HSPCs). Western blot was used to determine BCL-xL degradation and measure the expression of BCL-2 and MCL-1 after drug treatments.
RESULTS
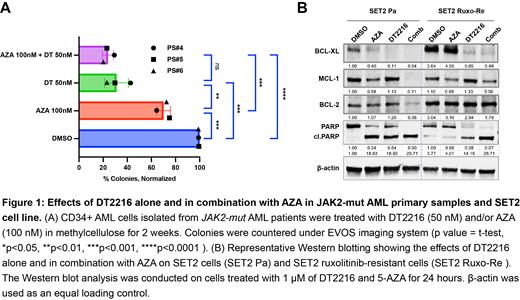
The analysis of BCL2L1 gene expression (p<0.05, JAK2-mut AML cell lines versus other AML cell lines; p<0.01, post-MPN AML versus de novo AML) and BCL2L1 CRISPR gene effect (p<0.05, JAK2-mut AML cell lines versus other AML cell lines) confirmed the role of BCL-xL as a target in JAK2-mut AML. DT2216 as a single agent significantly reduced cell viability in JAK2-mut AML cell lines, with an average IC 50 of 1.61 μM in parental cell lines (range 0.74 - 2.35 μM) and an average IC 50 of 5.37 μM in ruxo-re cell lines (range 1.29 - 8.99 μM) at 72 hours. Notably, DT2216 also markedly reduced cell viability in primary samples (n=3), with an average IC 50 value of 1.21 μM (range 0.41 - 2.59 μM) at 24 hours. Moreover, the average CIs of DT2216/S68425 combination, DT2216/AZA and DT2216/ruxolitinib combination were 0.13 (range 0.07 - 0.17), 0.17 (range 0.61 - 0.22), 0.36 (range 0.14 - 0.54), respectively at 72 hours. Clonogenic assays in three primary JAK2-mut AML samples demonstrated a 69% (range 58.3 - 77.5%) reduction of colony count when treated with DT2216 alone (50 nM) (p<0.001, versus DMSO group) and 76% (range 70.8 - 79.8%) reduction when treated with the combination of DT2216 (50 nM) with AZA (100 nM) (p<0.0001, versus DMSO group) (Fig. 1A). Western blot analysis demonstrated efficient degradation of the BCL-xL protein, with average reductions of 78.5% (range: 70 - 90%), 82.75% (range: 68 - 96%) (Fig. 1B), and 86.75% (range: 83 - 97%) after 24 hours of treatment with DT2216 alone or in combination with AZA or ruxolitinib in both the parental and ruxo-re SET2 and HEL cell lines.
CONCLUSIONS
These findings highlight the promising efficacy of DT2216 in JAK2-mut AML, as evidenced by reduced cell viability, on-target degradation of BCL-xL, and synergistic anti-leukemia effects when combined with AZA, ruxolitinib or MCL-1 inhibitor. These results provide valuable insights into future therapeutic strategies for the treatment of JAK2-mut AML, particularly in the context of post-MPN AML.
Disclosures
Zheng:Dialect Therapeutics: Current equity holder in private company, Other: Co-founder of and have equity in Dialectic Therapeutics, which develops BCL-XL and BCL-2 PROTACs to treat cancer., Patents & Royalties: Inventor of patent applications for use of BCL-XL and BCL-2 PROTACs as senolytic and antitumor agents., Research Funding. Gritsman:ADC Therapeutics: Research Funding; iOnctura: Research Funding. Bhalla:Foghorn Therapeutics Inc.: Research Funding. Pemmaraju:Imedex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ClearView Healthcare Partners: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Physician Education Resource (PER): Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; PeerView Institute for Medical Education: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; United States Department of Defense (DOD): Research Funding; National Institute of Health/National Cancer Institute (NIH/NCI): Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; OncLive: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Protagonist Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees; CancerNet: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CareDx: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Karger Publishers: Other: Licenses; ASCO Cancer.Net Editorial Board: Other: Leadership; Stemline: Consultancy, Membership on an entity's Board of Directors or advisory committees; Aplastic Anemia & MDS International Foundation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; ImmunoGen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pacylex: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; EUSA Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Neopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Curio Science: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Intellisphere: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Medscape: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Menarini Group: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Cimeio Therapeutics AG: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Patient Power: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Magdalen Medical Publishing: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ASH Committee on Communications: Other: Leadership; Harborside Press: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Dava Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Blueprint: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Aptitude Health: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; HemOnc Times/Oncology Times: Other: Uncompensated; Bristol Myers Squibb Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Zhou:Dialect Therapeutics: Current equity holder in private company, Other: Co-founder of and have equity in Dialectic Therapeutics, which develops BCL-XL and BCL-2 PROTACs to treat cancer. Member of Board of Director., Patents & Royalties: Inventor of patent applications for use of BCL-XL and BCL-2 PROTACs as senolytic and antitumor agents.. Tyner:Recludix Pharma: Membership on an entity's Board of Directors or advisory committees; Kronos: Research Funding; Meryx: Research Funding; Tolero: Research Funding; Schrodinger: Research Funding; Petra: Research Funding; Acerta: Research Funding; Aptose: Research Funding; AstraZeneca: Research Funding; Constellation: Research Funding; Genentech: Research Funding; Incyte: Research Funding. Konopleva:AbbVie, Forty Seven, Precision Biosciences, Gilead Sciences, Genentech, Janssen, Sanofi, MEI Pharma, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini.: Consultancy; Abbvie, Allogene Therapeutics, Cellectis, Forty Seven, Gilead Sciences, Genentech, Sanofi, MEI Pharma, Rafael Pharmaceuticals, Daiichi Sankyo Pharmaceutical, AstraZeneca Co., Menarini, Precision BioSciences.: Research Funding; Reata Pharmaceuticals.: Current holder of stock options in a privately-held company, Patents & Royalties.